MCAT General Chemistry Question 23: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 23
8. Which of the following is an important property of the group of elements shaded in the Periodic Table below?
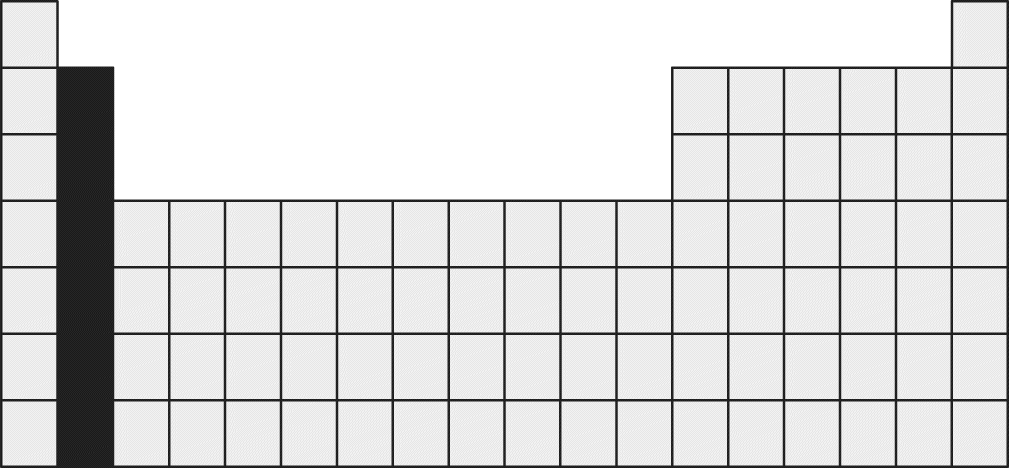
- A. These elements are the best electrical conductors in the Periodic Table.
- B. These elements form divalent cations.
- C. The second ionization energy for these elements is lower than the first ionization energy.
- D. The atomic radii of these elements decrease as one moves down the column.
Correct Answer: B
Explanation:
This block represents the alkaline earth metals, which form divalent cations, or ions with a +2 charge. All of the elements in Group IIA have two electrons in their outermost s subshell. Because loss of these two electrons would leave a full octet as the outermost shell, becoming a divalent cation is a stable configuration for all of the alkaline earth metals. Although some of these elements might be great conductors, they are not as effective as the alkali metals, eliminating choice (A). Choice (C) is also incorrect because, although forming a divalent cation is a stable configuration for the alkaline earth metals, the second ionization energy is still always higher than the first. Finally, choice (D) is incorrect because atomic radii increase when moving down a group of elements because the number of electron shells increases.
