MCAT General Chemistry Question 226: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 226
4. Na2SO4 is soluble in water. If NaCl(s) is added to a solution of Na2SO4(aq) so that the concentration of Na+ doubles, then the:
- A. solubility constant of Na2SO4 increases while that of NaCl decreases.
- B. solubility constants of Na2SO4 and NaCl both decrease.
- C. solubility of Na2SO4 and NaCl both decrease.
- D. solubility of Na2SO4 decreases while that of NaCl increases.
Correct Answer: C
Explanation:
C Solubility constants, like all equilibrium constants, are functions of temperature only. This eliminates choices A and B. Given the equilibria:
Na2SO4(s) 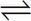 2 Na+ (aq) + SO2-4(aq)
2 Na+ (aq) + SO2-4(aq)
NaCl(s) 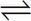 Na+ (aq) + Cl- (aq)
Na+ (aq) + Cl- (aq)
Na+ is a common ion to both systems. Increasing Na+ concentration will decrease the solubility of both salts, eliminating choice D and making choice C the best answer.
