MCAT General Chemistry Question 225: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 225
3. Given the following equilibrium:
N2(g) + 3 H2(g) 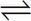 2 NH3(g) ∆H = -91.8kJ
2 NH3(g) ∆H = -91.8kJ
How would an increase in temperature affect the concentration of N2 at equilibrium?
- A. The concentration of N2 will increase because of an increase in Keq.
- B. The concentration of N2 will decrease because of an increase in Keq.
- C. The concentration of N2 will increase because of a decrease in Keq.
- D. The concentration of N2 will remain unchanged.
Correct Answer: C
Explanation:
C Since the reaction is exothermic, an increase in temperature will shift the equilibrium to the left, and the concentration of N2 will increase, eliminating choices B and D. For exothermic reactions, an increase in temperature will decrease the Keq, eliminating choice A and making choice C the correct answer.
