MCAT General Chemistry Question 224: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 224
2. A group of scientists is studying the dynamics of the acetic acid dissociation below and bring the process to equilibrium under standard conditions. If the scientists then add 35 g of sodium acetate to the reaction container, which of the following will be true?
CH3COOH(aq) 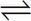 CH3COO-(aq) + H+ (aq)
CH3COO-(aq) + H+ (aq)
- A. Q > Keq and the reaction will move in reverse.
- B. Q < Keq and the reaction will move forward.
- C. Q > Keq and the reaction will move forward.
- D. Q < Keq and the reaction will move in reverse.
Correct Answer: A
Explanation:
A Keq = [products]/[reactants] when both reactant and product concentrations are those at equilibrium. Q = [products]/[reactants] regardless of whether reactant and product concentrations are those at equilibrium. The addition of sodium acetate essentially translates into the addition of acetate ion, a product in this equilibrium. As a result of such an addition, Q > Keq and products are present in excess of equilibrium values. Le Châtelier's principle states that net reverse movement is created when the concentration of products is increased in an equilibrium system.
