MCAT General Chemistry Question 223: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 223
1. Which of the following manipulations is capable of changing the Keq of the reaction shown below?
N2 (g) + H2(g) 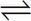 2 NH3(g)
2 NH3(g)
- A. Doubling the concentrations of N2 (g), H2(g), and NH3(g)
- B. Tripling the volume of the reaction container
- C. Increasing the pressure from 1 to 2 atm
- D. Decreasing the temperature to from 298 K to 273 K
Correct Answer: D
Explanation:
D Equilibrium constants are specific to a single temperature and standard state free energy change according to: ∆G° = -RTlnK. Altering temperature is the only answer choice that can change the reaction's Keq.
