MCAT General Chemistry Question 211: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 211
3. The figure below depicts the relative sizes and mole fractions of two monatomic gases in a closed container.
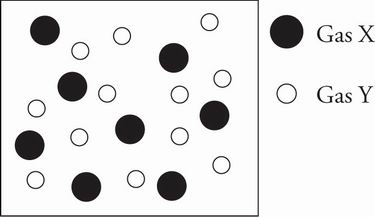
Which of the following is true about the gas mixture after a small hole is punched in the container and the gases are allowed to completely effuse?
- A. The partial pressure of Gas X will never equal the partial pressure of Gas Y.
- B. The partial pressure of Gas Y will decrease faster than the partial pressure of Gas X.
- C. The partial pressure of Gas Y will increase because the mole fraction of Gas Y will increase.
- D. The partial pressures of each gas will remain unchanged.
Correct Answer: B
Explanation:
B After a hole is punched in the container and the gases begin to escape, the total pressure of the container will decrease, and the individual partial pressures of the gases will therefore also decrease. Eliminate choices C and D. According to Graham's law, the lighter the gas molecule, the faster its rate of effusion through a small hole. Gas Y will effuse faster than Gas X, so its partial pressure will decrease at a faster rate. The gases are allowed to completely effuse and the figure indicates that the mole fraction of Y is slightly larger than X. Therefore there will most likely be a moment in time when the partial pressures of both gases are equal (and this will definitely be the case when the container is finally empty), eliminating choice A.
