MCAT General Chemistry Question 21: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 21
6. The properties of atoms can be predicted, to some extent, by their location within the Periodic Table. Which property or properties increase in the direction of the arrows shown?
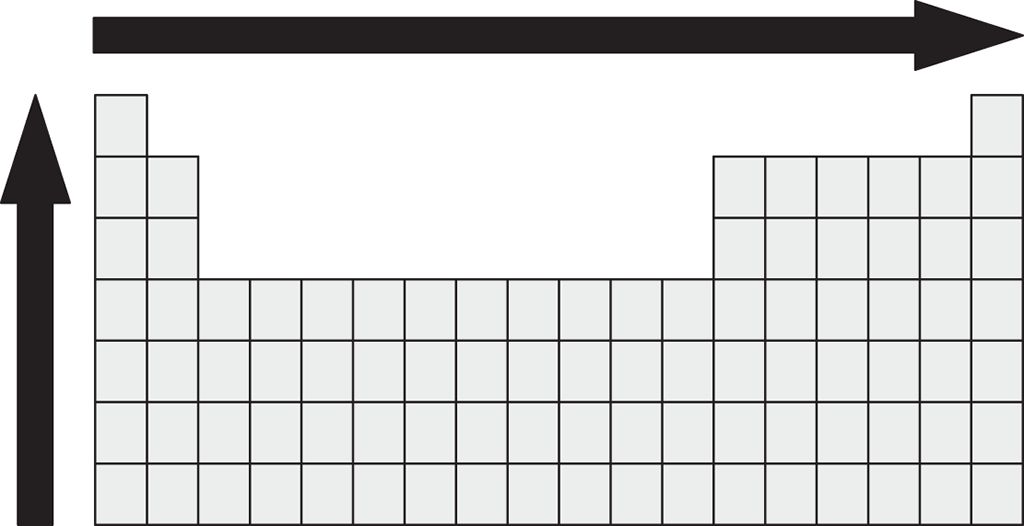
I. Electronegativity
II. Atomic radius
III. First ionization energy
- A. I only
- B. I and II only
- C. I and III only
- D. II and III only
Correct Answer: C
Explanation:
Electronegativity describes how strong an attraction an element will have for electrons in a bond. A nucleus with a larger effective nuclear charge will have a higher electro-negativity; Zeff increases toward the right side of a period. A stronger nuclear pull will also lead to increased first ionization energy, as the forces make it more difficult to remove an electron. The vertical arrow can be explained by the size of the atoms. As size decreases, the positive charge becomes more effective at attracting electrons in a chemical bond (higher electronegativity), and the energy required to remove an electron (ionization energy) increases.
