MCAT General Chemistry Question 2: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 2
2. Which of the following quantum number sets is possible?
- A. n = 2; l = 2; ml = 1;
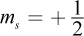
- B. n = 2; l = 1; ml = -1;
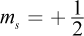
- C. n = 2; l = 0; ml = -1;
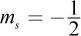
- D. n = 2; l = 0; ml = 1;
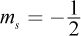
Correct Answer: B
Explanation:
The azimuthal quantum number l cannot be higher than n - 1, ruling out choice (A). The ml number, which describes the chemical's magnetic properties, can only be an integer value between -l and l. It cannot be equal to 1 if l = 0; this would imply that an s orbital has three subshells (-1, 0, and 1) when we know it can only have one. This rules out choices (C) and (D).
