MCAT General Chemistry Question 193: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 193
4. Which of the following best characterizes the spontaneous half-reaction below under standard conditions?
Pd2+ + 2e- → Pd
- A. NH3
- B. NH4+
- C. CO2
- D. CH4
Correct Answer: C
Explanation:
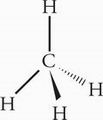
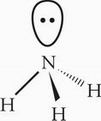
C The central atom, N, possesses three bonding electron groups and one lone pair of electrons. NH3 therefore has tetrahedral orbital geometry.
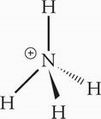
The central atom, N, possesses four bonding electron groups and zero lone pairs. NH4+ therefore has tetrahedral geometry.
The central atom, C, possesses two bonding electron groups and zero lone pairs. Recall that double bonds count as a single electron group. CO2 therefore has linear geometry.
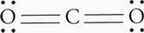
The central atom, C, possesses four bonding electron groups and zero lone pairs. CH4 therefore has tetrahedral geometry.