MCAT General Chemistry Question 192: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 192
3. The amino and carboxyl terminals of alanine lose protons according to the following equilibrium:
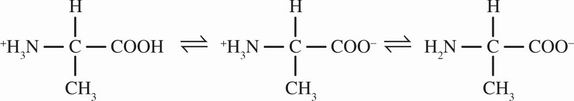
Which of the following indicators would be best used to determine the second equivalence point when alanine is titrated with sodium hydroxide?
- A. Ionic bonding
- B. Covalent bonding
- C. Van der Waals forces
- D. Induced dipole
Correct Answer: B
Explanation:
B Van der Waals forces and induced dipoles are both examples of intermolecular forces, not intramolecular bonding, therefore choices C and D can be eliminated. The disparity in electronegativities between the carbon (C) and nitrogen (N) atoms in cyanide is not sufficient enough to produce ionic bonding, therefore choice B, covalent bonding, is the best answer.

