MCAT General Chemistry Question 187: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 187
7. The figure below depicts the relative sizes and mole fractions of two monatomic gases in a closed container.
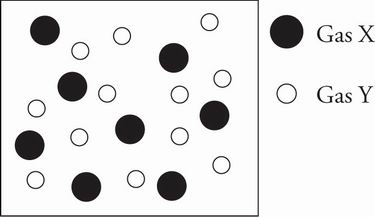
Which of the following is true about the gas mixture after a small hole is punched in the container and the gases are allowed to completely effuse?
- A. Na
- B. K
- C. Mg
- D. Ca
Correct Answer: D
Explanation:
D After their first ionizations, Na+ and K+ both have octet electron configurations, so a second ionization to remove another electron would require a very high amount of energy. This eliminates choices A and B. Ionization energy decreases down a group, due to increased nuclear shielding, so it is easier to remove electrons from Ca than Mg, making choice D the answer.
