MCAT General Chemistry Question 184: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 184
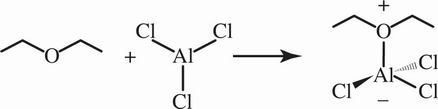
4. In the following reaction, which of the following most accurately describes the type of bond formed?
- A. Mg
- B. Ca
- C. Cr
- D. Zn
Correct Answer: C
Explanation:
C Diamagnetic atoms are repelled by magnetic fields and paramagnetic atoms are attracted to magnetic fields. Paramagnetic atoms have unpaired electrons in their valence orbitals. Mg and Ca are in the same group and have the same valence configuration, so both cannot be the right answer. Zn is at the end of the d block and has a valence shell with all of its electrons paired. Cr only has four electrons in its 3d subshell, resulting in four unpaired electron orbitals. Cr is the only choice that is paramagnetic and would be attracted to a magnetic field.
