MCAT General Chemistry Question 181: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 181
1. When an atom of plutonium-239 is bombarded with an alpha particle, this element along with one free neutron is created:
- A. Californium-240
- B. Californium-241
- C. Curium-242
- D. Curium-243
Correct Answer: C
Explanation:
C The process described is transmutation, and the new nucleus can be determined by writing a balanced nuclear equation. The preliminary equation to balance is this:
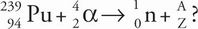
where the question mark stands for the new element formed. Balancing mass number gives 239 + 4 = 1 + A, where A = 242; balancing the atomic number gives 94 + 2 = 0 + Z, where Z = 96. Therefore, element number 96 is curium (eliminate choices A and B), and the appropriate isotope has a mass number of 242.
