MCAT General Chemistry Question 175: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 175
10. Which of the following expressions correctly describes the relationship between standard electromotive force and standard change in free energy?
- A. ΔG° = -nF(E°red,anode - E°red,cathode)
- B.
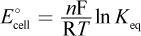
- C.
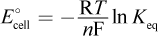
- D. ΔG° = nF(E°red,anode - E°red,cathode)
Correct Answer: D
Explanation:
There are only two equations involving standard change in free energy in electrochemical cells: ΔG° = -nFE°cell and ΔG° = -RT ln Keq. Substituting E°cell = E°red,cathode - E°red,anode into the first equation and distributing the negative sign gives choice (D). Choice (A) would be the opposite of ΔG°. Setting the two equations equal to each other, we get RT ln Keq = nFE°cell. Solving for E°cell, we get 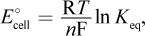 which is the opposite of choice (B). Choice (C) incorrectly solves the algebra.
which is the opposite of choice (B). Choice (C) incorrectly solves the algebra.
