MCAT General Chemistry Question 174: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 174
9. Which of the following statements could be true about a Na–Cd cell, based on the information below?
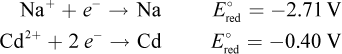
- A. It is a galvanic cell, and sodium is the cathode.
- B. It is an electrolytic cell, and cadmium is the anode.
- C. It is a galvanic cell, with E°cell = 3.11 V.
- D. It is an electrolytic cell, with E°cell = -3.11 V.
Correct Answer: B
Explanation:
If this were a galvanic cell, the species with the more positive reduction potential (cadmium) would be reduced. The cathode is always reduced in an electrochemical cell, so sodium could not be the cathode in such a galvanic cell, eliminating choice (A). Sodium would be the cathode in an electrolytic cell, however, which would make cadmium the anode. Thus, the answer is choice (B). Note that we do not have to determine E°cell because we already know the answer. However, the E°cell would be -2.71 - (-0.40) = -2.31 V for an electrolytic cell, and +2.31 V for a galvanic cell, eliminating choices (C) and (D).
