MCAT General Chemistry Question 170: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 170
5. If the value of E°cell is known, what other data is needed to calculate ΔG°?
- A. Equilibrium constant
- B. Reaction quotient
- C. Temperature of the system
- D. Half-reactions of the cells
Correct Answer: D
Explanation:
This answer comes directly from the equation relating Gibbs free energy and E°cell. ΔG° = -nFE°cell, where n is the number of moles of electrons transferred and F is the Faraday constant, 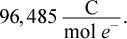 To determine n, one must look at the balanced half-reactions occurring in the oxidation-reduction reaction.
To determine n, one must look at the balanced half-reactions occurring in the oxidation-reduction reaction.
