MCAT General Chemistry Question 168: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 168
3. Consider the following data:
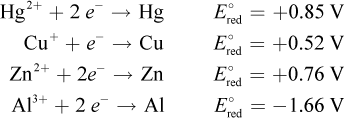
The anode of a certain galvanic cell is composed of copper. Which of the metals from the data table can be used at the cathode, assuming equal concentrations of the two electrolyte solutions?
- A. Hg
- B. Cu
- C. Zn
- D. Al
Correct Answer: A
Explanation:
Oxidation occurs at the anode, and reduction occurs at the cathode. Because Cu is the anode, it must be oxidized. The reduction potential of the cathode cannot be less than that of the anode for a galvanic cell. Therefore, mercury, choice (A), must be the cathode. In a concentration cell, the same material is used as both the cathode and anode; however, this question assumes equal concentrations. If both electrolyte solutions have the same concentration, there will be no oxidation-reduction reaction and, therefore, no anode or cathode. This eliminates choice (B).
