MCAT General Chemistry Question 167: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 167
2. Given the following standard reduction potentials:
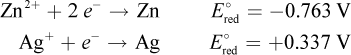
What is the standard electromotive force of the following reaction?
Zn2+ + 2 Ag → 2 Ag+ + Zn
- A. –2.2 V
- B. –1.1 V
- C. +1.1 V
- D. +2.2 V
Correct Answer: B
Explanation:
To determine the standard electromotive force of a cell, simply subtract the standard reduction potentials of the two electrodes. In this case, the cathode is zinc because it is being reduced; the anode is silver because it is being oxidized. Thus,
E°cell = E°red,cathode - E°red,anode = - 0.763 - 0.337 = - 1.10 V
While we must multiply the silver half-reaction by two to balance electrons, the actual value for the reduction potential does not change. Remember that the standard reduction potential is determined by the identity of the electrode, not the amount of it present.
