MCAT General Chemistry Question 165: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 165
15. An assay is performed to determine the gold content in a supply of crushed ore. One method for pulling gold out of ore is to react it in a strong cyanide (CN–) solution. The equation is provided below:
Au + NaCN + O2 + H2O → Na[Au(CN)2] + NaOH
An indicator is used during this reaction, and approximately 100 mL of a 2 M NaCN solution is used to reach the endpoint. How many moles of Au are present in the crushed ore?
- A. 0.01 mol
- B. 0.02 mol
- C. 0.10 mol
- D. 0.20 mol
Correct Answer: C
Explanation:
First, balance the chemical equation:4 Au + 8 NaCN + O2 + 2 H2O → 4 Na[Au(CN)2] + 4 NaOH
Now, determine the number of moles of NaCN used in the reaction:
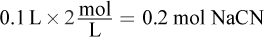
If 0.2 mol NaCN are used in the reaction, then 0.2 mol
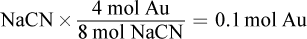
is oxidized.
