MCAT General Chemistry Question 164: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 164
14. After balancing the following oxidation–reduction reaction, what is the sum of the stoichiometric coefficients of all of the reactants and products?
S8 (s) + NO3- (aq) → SO32- (aq) + NO (g)
- A. 4
- B. 50
- C. 91
- D. 115
Correct Answer: D
Explanation:
Utilize the method described earlier to balance this redox reaction. The balanced half-reactions are:
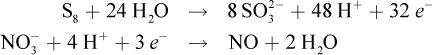
To get equal numbers of electrons in each half-reaction, the oxidation half-reaction will have to be multiplied by 3, and the reduction half-reaction will have to be multiplied by 32:
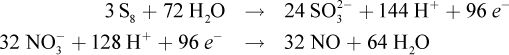
This makes the overall reaction:3S8 + 32 NO3- + 8 H2O → 24 SO32- + 32 NO + 16 H+
The sum of the stoichiometric coefficients is therefore 3 +32 + 8 + 24 + 32 + 16 = 115.
