MCAT General Chemistry Question 159: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 159
9. As methanol is converted to methanal, and then methanoic acid, the oxidation number of the carbon:
- A. increases.
- B. decreases.
- C. increases, then decreases.
- D. decreases, then increases.
Correct Answer: A
Explanation:
The formula for methanol is H3COH, for methanal is H2CHO, and for methanoic acid is HCOOH. If we assign oxidation numbers to carbon in each molecule, it starts at -2, then becomes 0, then becomes +2:
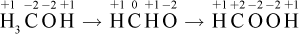
In general, it is often easier to think of oxidation as a gain of bonds to oxygen (or a similarly electronegative element) or loss of bonds to hydrogen for organic compounds. Therefore, because the carbon is oxidized as one converts from an alcohol to an aldehyde to a carboxylic acid, the oxidation number must increase.
