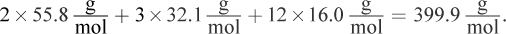MCAT General Chemistry Question 157: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 157
7. One way to test for the presence of iron in solution is by adding potassium thiocyanate to the solution. The product when this reagent reacts with iron is FeSCN2+, which creates a dark red color in solution via the following net ionic equation:
Fe3+ + SCN– → FeSCN2+
How many grams of iron sulfate would be needed to produce 2 moles of FeSCN2+?
- A. 110 g
- B. 220 g
- C. 400 g
- D. 500 g
Correct Answer: C
Explanation:
What you are shown is a net ionic equation. If two moles of FeSCN are created, two moles of Fe3+ must be used because the mole ratio is 1:1. Read the question stem carefully—we are asked for the mass of iron sulfate that is necessary, not the amount of iron, which would be 111.6 g, choice (A). Iron sulfate has the formula Fe2(SO4)3 because sulfate has a charge of -2 and iron has a charge of +3 (based on the net ionic equation). Therefore, one mole of iron sulfate is needed to make two moles of iron for the reaction. The molar mass of iron sulfate is