MCAT General Chemistry Question 146: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 146
11. What is the [H3O+] of a 2 M aqueous solution of a weak acid HXO2 with Ka = 3.2 × 10?5?
- A. 6.4 × 10?5 M
- B. 1.3 × 10?4 M
- C. 4.0 × 10?3 M
- D. 8.0 × 10?3 M
Correct Answer: D
Explanation:
This question requires the application of the acid dissociation formula. Weak acids do not dissociate completely; therefore, all three species that appear in the balanced equation will be present in solution. Hydrogen ions and conjugate base anions dissociate in equal amounts, so [H+] = [XO2-]. If the initial concentration of HXO2 was 2 M and some amount x dissociates, we will have x amount of H3O+ and XO2- at equilibrium, with 2 M - x amount of HXO2 at equilibrium.
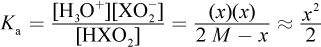
Note that x was considered negligible when added or subtracted, per usual. Solving for x, we get: