MCAT General Chemistry Question 145: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 145
10. What is the gram equivalent weight of phosphoric acid?
- A. 24.5 g
- B. 32.7 g
- C. 49.0 g
- D. 98.0 g
Correct Answer: B
Explanation:
Gram equivalent weight is the weight (in grams) that releases 1 acid or base equivalent from a compound. Because H3PO4 contains 3 protons, we find the gram equivalent weight by dividing the mass of one mole of the species by 3. The molar mass of phosphoric acid is 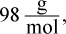 so the gram equivalent weight is 32.7 g.
so the gram equivalent weight is 32.7 g.
