MCAT General Chemistry Question 141: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 141
6. What is the pH of a solution with an ammonium concentration of 70 mM and an ammonia concentration of 712 mM? (Note: The pKb of ammonia is 3.45.)
- A. 2.45
- B. 4.45
- C. 9.55
- D. 11.55
Correct Answer: D
Explanation:
The question is asking for pH, but because of the information given, we must first find the pOH and then subtract it from 14 to get the pH. Use the Henderson-Hasselbalch equation:
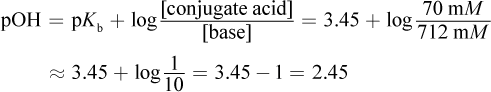
If the pOH = 2.45, the pH = 14 - 2.45 = 11.55.
