MCAT General Chemistry Question 136: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 136
1. Which of the following is not a Bronsted–Lowry base?
- A.
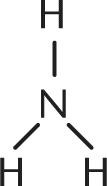
- B. F-
- C.
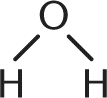
- D. H — O — N = O
Correct Answer: D
Explanation:
A Bronsted-Lowry base is defined as a proton acceptor. Ammonia, fluoride, and water—choices (A), (B), and (C), respectively—each accept a proton. Choice (D), HNO2, is a far better Bronsted-Lowry acid, donating a proton to solution.
