MCAT General Chemistry Question 135: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 135
15. The following equilibrium exists when AgBr (Ksp = 7.7 × 10-13) is in solution:
AgBr (s) ←→ Ag+ (aq) + Br- (aq)What is the solubility of AgBr in a solution of 0.0010 M NaBr?
- A.
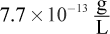
- B.
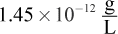
- C.
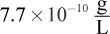
- D.
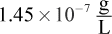
Correct Answer: D
Explanation:
The solubility of AgBr can be determined using the Ksp value given in the equation. Some amount of AgBr will dissolve. If we call this amount x, then there will be x amount of silver(I) formed and x amount of bromide—which is added to the 0.0010 M already present from NaBr.
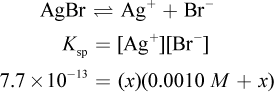
Remember that x is always very small. Even though 0.0010 M is also very small, it will still be much larger than the value of x on Test Day. Thus, the math can be simplified to: 7.7 × 10-13 = (x)(0.001). Therefore, x, the molar solubility, is 7.7 × 10-10, which looks like choice (C). However, the units are grams per liter, not molarity. Thus, we must multiply by the molar mass 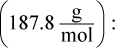
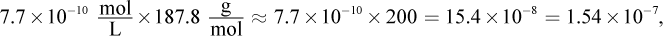
which is close to choice (D).
