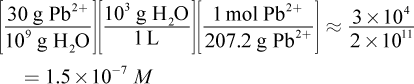MCAT General Chemistry Question 133: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 133
13. Lead is a toxic element that can cause many symptoms, including mental retardation in children. If a body of water is polluted with lead ions at 30 ppb (parts per billion), what is the concentration of lead expressed as molarity? (Note: The density of water is 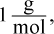 and ppb = grams per 109 grams of solution)
and ppb = grams per 109 grams of solution)
- A. 1.4 × 10-10 M Pb2+
- B. 1.4 × 10-7 M Pb2+
- C. 6.2 × 10-7 M Pb2+
- D. 6.2 × 10-6 M Pb2+
Correct Answer: B
Explanation:
30 ppb of Pb2+ is equivalent to 30 grams of Pb2+ in 109 grams of solution; given the extremely low concentration of lead, we can assume the mass of the water is around 109 grams, as well. From here, this is simply a dimensional analysis question. The units we want at the end are moles per liter (molarity), so we must covert from grams of lead to moles of lead and grams of water to liters of water: