MCAT General Chemistry Question 128: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 128
8. One hundred grams of sucrose are dissolved in a cup of hot water at 80°C. The cup of water contains 300.00 mL of water. What is the percent composition by mass of sugar in the resulting solution?
(Note: Sucrose = C12H22O11, density of water at 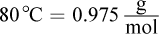 )
)
- A. 25.0%
- B. 25.5%
- C. 33.3%
- D. 34.2%
Correct Answer: B
Explanation:
The mass percent of a solute equals the mass of the solute divided by the mass of the total solution times 100%. To find the mass of the solution, first find the mass of the solvent, water. Multiplying the volume of the water by the density gives a mass of 292.5 grams of water. Adding 100 grams of sugar yields a solution with a mass of 392.5 grams. Next, divide 100 grams of sugar by 392.5 grams and multiply by 100 to get 25.5%. Choice (A) results if water's density at 80°C is assumed to be 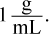 If we had forgotten to add the solute's mass to the solvent's, we would have calculated 34.2%, which is choice (D). Choice (C) neglects both the addition step and the correct density value.
If we had forgotten to add the solute's mass to the solvent's, we would have calculated 34.2%, which is choice (D). Choice (C) neglects both the addition step and the correct density value.
