MCAT General Chemistry Question 121: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 121
1. An aqueous solution was prepared by mixing 70 g of an unknown nondissociating solute into 100 g of water. The solution has a boiling point of 101.11°C. What is the molar mass of the solute?
(Note:)
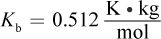
- A.
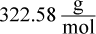
- B.
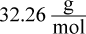
- C.
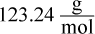
- D.
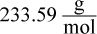
Correct Answer: A
Explanation:
The equation ΔTb = iKbm can be used to solve this problem. The change in boiling point is 101.11 - 100 = 1.11°C. Then, we can plug that into:
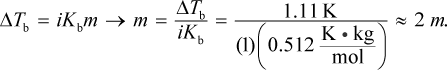
The van 't Hoff factor for this solute is 1 because the molecule does not dissociate into smaller components. Then, we can convert to grams of solute using the definition of molality:
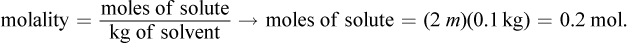
The mass used in this equation is 0.1 kg because 100 mL of water has a mass of 0.1 kg. Then, we can determine the molar mass:
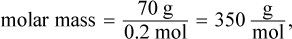
which is closest to choice (A).
