MCAT General Chemistry Question 114: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 114
9. A balloon at standard temperature and pressure contains 0.20 moles of oxygen and 0.60 moles of nitrogen. What is the partial pressure of oxygen in the balloon?
- A. 0.20 atm
- B. 0.25 atm
- C. 0.33 atm
- D. 0.80 atm
Correct Answer: B
Explanation:
At STP, the pressure inside the balloon equals 1 atm. The total number of moles in the balloon equals 0.20 moles plus 0.60 moles, or 0.80 moles. PO2 equals the mole fraction of oxygen 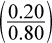 times the total pressure, 1 atm. The partial pressure of oxygen is therefore 0.25 atm.
times the total pressure, 1 atm. The partial pressure of oxygen is therefore 0.25 atm.
