MCAT General Chemistry Question 113: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 113
8. The plots of two gases at STP are shown below. One of the gases is 1.0 L of helium, and the other is 1.0 L of bromine. Which plot corresponds to each gas and why?
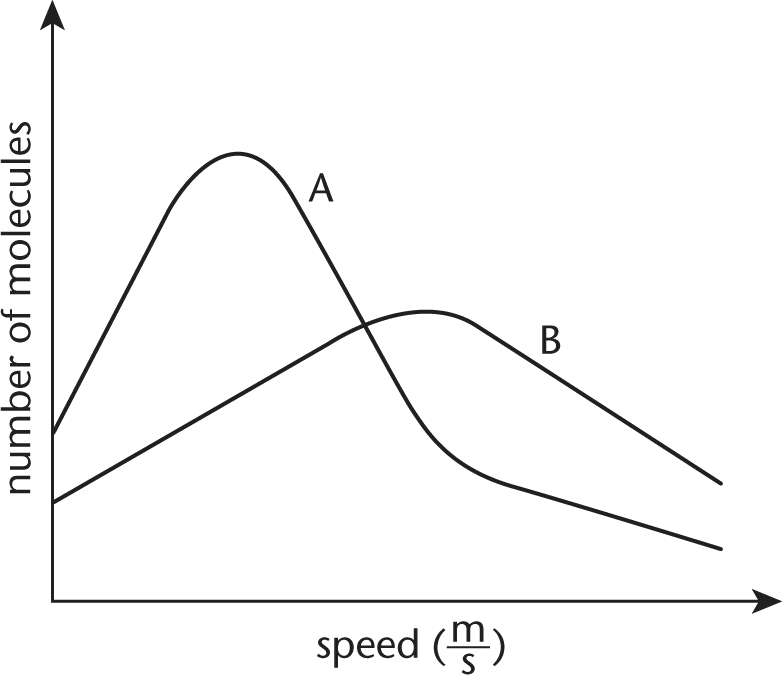
- A. Curve A is helium and curve B is bromine because helium has a smaller molar mass than bromine.
- B. Curve A is helium and curve B is bromine because the average kinetic energy of bromine is greater than the average kinetic energy of helium.
- C. Curve A is bromine and curve B is helium because helium has a smaller molar mass than bromine.
- D. Curve A is bromine and curve B is helium because the average kinetic energy of bromine is greater than the average kinetic energy of helium.
Correct Answer: C
Explanation:
At STP, the difference between the distribution of speeds for helium and bromine gas is due to the difference in molar mass. Helium has a smaller molar mass than bromine. Particles with small masses travel faster than those with large masses, so the helium gas corresponds to curve B, which has a higher average speed. Because the gases are at the same temperature (273 K), they have the same average kinetic energy, eliminating choices (B) and (D).
