MCAT General Chemistry Question 111: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 111
6. An 8.00 g sample of NH4NO3 (s) is placed into an evacuated 10 L flask and heated to 227°C. After the NH4NO3 completely decomposes, what is the approximate pressure in the flask?
NH4NO3 (s) → N2O (g) + H2O (g)
- A. 0.410 atm
- B. 0.600 atm
- C. 0.821 atm
- D. 1.23 atm
Correct Answer: D
Explanation:
The first thing to do is balance the given chemical equation: NH4NO3 (s) → N2O (g) + 2 H2O (g). The mass given is 8.00 g, which represents 0.1 mol NH4NO3 (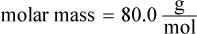 ). When 0.1 mol of the solid decomposes, it will form 0.1 mol N2O and 0.2 mol water. This gives approximately 0.3 moles of gas product. The ideal gas equation can be used to obtain the pressure in the flask:
). When 0.1 mol of the solid decomposes, it will form 0.1 mol N2O and 0.2 mol water. This gives approximately 0.3 moles of gas product. The ideal gas equation can be used to obtain the pressure in the flask:
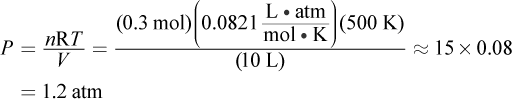
Choice (C) is the result if one assumes the equation is balanced, obtaining 0.2 mol gas as the product.
