MCAT General Chemistry Question 11: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 11
11. Suppose that an atom fills its orbitals as shown:
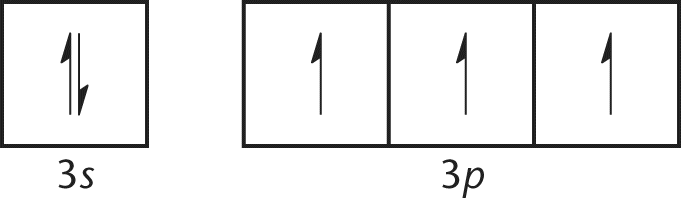
Such an electron configuration most clearly illustrates which of the following laws of atomic physics?
- A. Hund's rule
- B. Heisenberg uncertainty principle
- C. Bohr model
- D. Rutherford model
Correct Answer: A
Explanation:
The MCAT covers the topics in this chapter qualitatively more often than quantitatively. It is critical to be able to distinguish the fundamental principles that determine electron organization, which are usually known by the names of the scientists who discovered or postulated them. The Heisenberg uncertainty principle, choice (B), refers to the inability to know the momentum and position of a single electron simultaneously. The Bohr model, choice (C), was an early attempt to describe the behavior of the single electron in a hydrogen atom. The Rutherford model, choice (D), described a dense, positively charged nucleus. The element shown here, nitrogen, is often used to demonstrate Hund's rule because it is the smallest element with a half-filled p subshell. Hund's rule explains that electrons fill empty orbitals first before doubling up electrons in the same orbital.
