MCAT General Chemistry Question 108: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 108
3. A leak of helium gas through a small hole occurs at a rate of 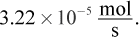 How will the leakage rates of neon and oxygen gases compare to helium at the same temperature and pressure?
How will the leakage rates of neon and oxygen gases compare to helium at the same temperature and pressure?
- A. Neon will leak faster than helium; oxygen will leak faster than helium.
- B. Neon will leak faster than helium; oxygen will leak slower than helium.
- C. Neon will leak slower than helium; oxygen will leak faster than helium.
- D. Neon will leak slower than helium; oxygen will leak slower than helium.
Correct Answer: D
Explanation:
Graham's law of effusion states that the relative rates of effusion of two gases at the same temperature and pressure are given by the inverse ratio of the square roots of the masses of the gas particles. In other words, a gas with a higher molar mass will leak more slowly than a gas with a lower molar mass. Both neon and oxygen gases will leak at slower rates than helium because they both have more mass more than helium.
