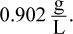MCAT General Chemistry Question 107: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 107
2. What is the density of neon gas in 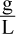 at STP?
at STP?
- A. 452.3
- B. 226.0
- C. 1.802
- D. 0.9018
Correct Answer: D
Explanation:
Density equals mass divided by volume. The mass of 1 mole of neon gas equals 20.2 grams. At STP, 1 mole of neon occupies 22.4 L. Dividing the mass, 20.2 grams, by the volume, 22.4 L, gives an approximate density of