MCAT General Chemistry Question 103: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 103
13. A reaction coordinate for a chemical reaction is displayed in the graph below.
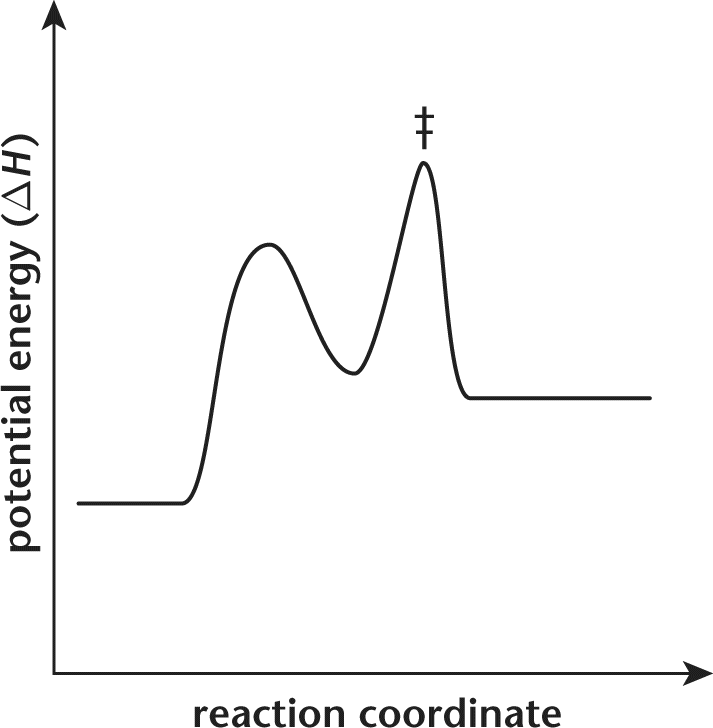
Which of the following terms describes the energy of this reaction?
- A. Endothermic
- B. Exothermic
- C. Endergonic
- D. Exergonic
Correct Answer: A
Explanation:
Eliminate choices (C) and (D), which describe the free energy of reaction and cannot be determined from this graph. While most reaction coordinate graphs we've explored in this book use free energy for the y-axis, this one uses potential energy (enthalpy). If the heat of formation of the products is greater than that of the reactants, the reaction is endothermic. We can determine this information from their relative positions on the graph: because the products are higher than the reactants, this is an endothermic reaction.
