MCAT Biochemistry Question 167: Answer and Explanation
Home > MCAT Test > MCAT biochemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT biochemistry practice tests.
Question: 167
2. At 25°C the ΔG° for a certain reaction A → B + 2 C is 0. If the concentration of A, B, and C in the cell at 25°C are all 10 mM, how does the ΔG compare to the measurement taken with 1 M concentrations?
- A. ΔG is greater than ΔG°, thus the reaction is spontaneous.
- B. ΔG is less than ΔG°, thus the reaction is spontaneous.
- C. ΔG is greater than ΔG°, thus the reaction is nonspontaneous.
- D. ΔG is less than ΔG°, thus the reaction is nonspontaneous.
Correct Answer: B
Explanation:
To solve this question, we can use the equation ΔG = ΔG° + RT ln Q. Q, the reaction quotient, is 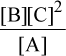 for this reaction. Plugging in the variables, we get ΔG = 0 + RT
for this reaction. Plugging in the variables, we get ΔG = 0 + RT
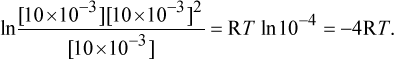
Because both R and T are positive, we know that ΔG must be negative and therefore lower than the original value. A negative ΔG corresponds to a spontaneous reaction.
